Difference between revisions of "Fluorescence Lifetime Imaging Microscopy Quantitative Measurements"
| Line 28: | Line 28: | ||
| − | The acquisition of FD FLIM measurements is illustrated using the ISS Alba Fast FLIM system (ISS Inc., Champaign, IL) coupled to an Olympus IX71 microscope (Figure 2B). The microscope is equipped with a 60X 1.2 numerical aperture water-immersion objective lens, an objective warmer, and a stage-top environmental control system to maintain the temperature and <chem>CO2</chem> levels of the sample. A 5 mW 440 nm diode laser was modulated by the Alba Fast FLIM system at a fundamental frequency of 10 MHz, with additional measurements at 13 harmonics (20-140 MHz). The modulated laser is coupled to the confocal scanning system that is controlled by the Vista Vision software (ISS Inc., Champaign, IL). The fluorescence signals emitted from the specimen are routed by a 495 nm long-pass beam splitter to two separate detection channels using band-pass emission filters (here, 480/40 nm and 530/43 nm) and the signals are detected using two identical avalanche photodiodes (APD) (Figure 2B). | + | The acquisition of FD FLIM measurements is illustrated using the ISS Alba Fast FLIM system (ISS Inc., Champaign, IL) coupled to an Olympus IX71 microscope (Figure 2B). The microscope is equipped with a 60X 1.2 numerical aperture water-immersion objective lens, an objective warmer, and a stage-top environmental control system to maintain the temperature and <chem>CO2</chem> <chem>H2O</chem> levels of the sample. A 5 mW 440 nm diode laser was modulated by the Alba Fast FLIM system at a fundamental frequency of 10 MHz, with additional measurements at 13 harmonics (20-140 MHz). The modulated laser is coupled to the confocal scanning system that is controlled by the Vista Vision software (ISS Inc., Champaign, IL). The fluorescence signals emitted from the specimen are routed by a 495 nm long-pass beam splitter to two separate detection channels using band-pass emission filters (here, 480/40 nm and 530/43 nm) and the signals are detected using two identical avalanche photodiodes (APD) (Figure 2B). |
Revision as of 07:47, 16 February 2017
Department of Cellular and Integrative Physiology, Indiana University School of Medicine, 635 Barnhill Dr., Indianapolis, IN 46202 USA.
1. A Brief History of the Measurement of Fluorescence Lifetimes
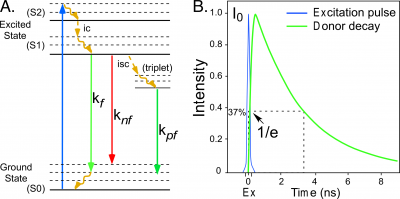
Fluorescence describes the emission of light by an atom or molecule that follows the absorption of electromagnetic energy [1]. When a fluorescent molecule absorbs energy, it is driven into an excited state that persists for a brief time. The molecule then transitions back to the lower energy ground state by one of several possible pathways. Some of the pathways for de-excitation to the ground state are illustrated in the simplified Jabłoński-Perrin diagram in Figure 1. The pathways include internal conversion ([math]ic[/math]), decay by fluorescence ([math]kf[/math]), quenching (loss of excitation energy without the emission of light, [math]knf[/math]), or intersystem crossing ([math]isc[/math]) to the triplet state followed by decay by phosphorescence ([math]kpf[/math]).The average time required for a population of fluorophores in the excited state to decay to the ground state is called the fluorescence lifetime, which is described by an exponential function (Figure 1):
[math]I(t)=I_{0}e^{-t/\tau}\tag 1[/math]
where [math]I(t)[/math] is the fluorescence impulse response at time [math]t[/math], [math]I_{0}[/math] is the initial intensity after the excitation pulse, and [math]\tau[/math] is the fluorescence lifetime.
The measurement of excited-state lifetimes began in the mid-eighteenth century with the studies of phosphorescent materials by Alexandre-Edmond Becquerel, who was the first to measurethe exponential decay of phosphorescence over time [2, 3]. In the early 1920’s Robert Wood illuminated a heated vapor of mercury and demonstrated that the emission of light was displaced in the moving vapor relative to the excitation spot. He then used the velocity of the vapor to determine the lifetime [4].Two years later, Phillip Gottling used an improved version of Wood’s approach to determine that the excited-state lifetime of rhodamine was twenty nanoseconds. He proposed that the “exciting energy was imprisoned for a short but definite and measurable interval of time” within the fluorescent molecule [5].The excited-state events observed by Becquerel, Wood, and Gottling are explained by the Bohr model of the atom, which describes how the absorption of energy causes electrons to move from inner to outer stable orbits, with their return to the original orbit being coupled to the emission of quanta of light [6].
In 1926, Enrique Gaviola developed the phase fluorometer, an instrument designed specifically for lifetime measurements. Gaviola's fluorometer was the first instrument that could accurately measure nanosecond fluorescence lifetime decays [7]. This enabled Gaviola to demonstrate how events in the probe environment competing in the deactivation from the excited state (Figure 1) cause the fluorescence lifetime to change. Therefore, the measured fluorescence lifetime ([math]\tau_{f}[/math]) represents the combination of the radiative ([math]kf[/math]) and non-radiative ([math]knf[/math]) decay rates for the transition from the excited state:
[math]tau_{f}=\frac{1}{k_{f}+k_{nf}}\tag 2[/math]
Fluorescence lifetime imaging microscopy (FLIM) is a technique that can be used to quantify the duration of the excited state, and thus quantify events that affect the excited state of a fluorophore. FLIM detects the changes in fluorescence lifetimes on the time scales of nanoseconds, and is routinely used to measure protein interactions or signaling events as they occur within living cells.
2. Frequency Domain (FD) FLIM
The FLIM techniques are broadly subdivided into time domain and frequency domain (FD) methods. The physics that underlies these two methods is identical, but they differ in how the signals are analyzed [8]. The frequency domain (FD) FLIM approach described here excites the fluorophores with a light source that is modulated at high frequencies, typically between 10 to 300 megahertz (MHz).Since the excitation source is modulated, the emission from the fluorophores will also be modulated. However, because of the persistence of the excited state there will be a phase delay ([math]\Phi[/math]) and a change in the modulation ([math]M[/math]) of the emission signal relative to the corresponding excitation waveform (Figure 2A).The fluorescence lifetime([math]\tau_{f}[/math]) can be determined directly from the [math]\Phi_{\tau_{p}}[/math] and the [math]M_{\tau_{m}}[/math] of the emission signal for each excitation frequency:
[math] \tau_{p}=\tan (\Phi ) and \tau_{m}=\sqrt{\frac{1}{M^2}-1}\tag 3[/math]
The acquisition of FD FLIM measurements is illustrated using the ISS Alba Fast FLIM system (ISS Inc., Champaign, IL) coupled to an Olympus IX71 microscope (Figure 2B). The microscope is equipped with a 60X 1.2 numerical aperture water-immersion objective lens, an objective warmer, and a stage-top environmental control system to maintain the temperature and <chem>CO2</chem> <chem>H2O</chem> levels of the sample. A 5 mW 440 nm diode laser was modulated by the Alba Fast FLIM system at a fundamental frequency of 10 MHz, with additional measurements at 13 harmonics (20-140 MHz). The modulated laser is coupled to the confocal scanning system that is controlled by the Vista Vision software (ISS Inc., Champaign, IL). The fluorescence signals emitted from the specimen are routed by a 495 nm long-pass beam splitter to two separate detection channels using band-pass emission filters (here, 480/40 nm and 530/43 nm) and the signals are detected using two identical avalanche photodiodes (APD) (Figure 2B).