Difference between revisions of "Fluorescence Lifetime Imaging Microscopy Quantitative Measurements"
| Line 5: | Line 5: | ||
---- | ---- | ||
'''1. A Brief History of the Measurement of Fluorescence Lifetimes''' | '''1. A Brief History of the Measurement of Fluorescence Lifetimes''' | ||
| − | + | [[File:RDayFig1_copy.png|thumb|400px|Figure 1.|Figure 1. .(A) A simplified Perrin-Jabłoński plot illustrating the decay from the excited state to the ground state by radiative (<math>kf, kpf</math>) and non-radiative (<math>knf</math>) pathways. (B) The exponential decay of fluorescence from a population of molecules illuminated by a brief excitation pulse.]] | |
[[Fluorescence]] describes the emission of light by an atom or molecule that follows the absorption of electromagnetic energy [1]. When a fluorescent molecule absorbs energy, it is driven into an excited state that persists for a brief time. The molecule then transitions back to the lower energy ground state by one of several possible pathways. Some of the pathways for de-excitation to the ground state are illustrated in the simplified [[Jabłoński-Perrin diagram]] in Figure 1. The pathways include internal conversion (<math>ic</math>), decay by fluorescence (<math>kf</math>), quenching (loss of excitation energy without the emission of light, <math>knf</math>), or [[intersystem crossing]] (<math>isc</math>) to the [[triplet state]] followed by decay by [[phosphorescence]] (<math>kpf</math>).The average time required for a population of fluorophores in the excited state to decay to the ground state is called the [[fluorescence lifetime]], which is described by an exponential function (Figure 1): | [[Fluorescence]] describes the emission of light by an atom or molecule that follows the absorption of electromagnetic energy [1]. When a fluorescent molecule absorbs energy, it is driven into an excited state that persists for a brief time. The molecule then transitions back to the lower energy ground state by one of several possible pathways. Some of the pathways for de-excitation to the ground state are illustrated in the simplified [[Jabłoński-Perrin diagram]] in Figure 1. The pathways include internal conversion (<math>ic</math>), decay by fluorescence (<math>kf</math>), quenching (loss of excitation energy without the emission of light, <math>knf</math>), or [[intersystem crossing]] (<math>isc</math>) to the [[triplet state]] followed by decay by [[phosphorescence]] (<math>kpf</math>).The average time required for a population of fluorophores in the excited state to decay to the ground state is called the [[fluorescence lifetime]], which is described by an exponential function (Figure 1): | ||
| Line 11: | Line 11: | ||
where <math>I(t)</math> is the fluorescence impulse response at time <math>t</math>, <math>I_{0}</math> is the initial intensity after the excitation pulse, and <math>\tau</math> is the fluorescence lifetime. | where <math>I(t)</math> is the fluorescence impulse response at time <math>t</math>, <math>I_{0}</math> is the initial intensity after the excitation pulse, and <math>\tau</math> is the fluorescence lifetime. | ||
| − | |||
Revision as of 07:00, 16 February 2017
Department of Cellular and Integrative Physiology, Indiana University School of Medicine, 635 Barnhill Dr., Indianapolis, IN 46202 USA.
1. A Brief History of the Measurement of Fluorescence Lifetimes
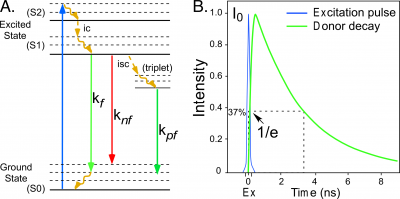
Fluorescence describes the emission of light by an atom or molecule that follows the absorption of electromagnetic energy [1]. When a fluorescent molecule absorbs energy, it is driven into an excited state that persists for a brief time. The molecule then transitions back to the lower energy ground state by one of several possible pathways. Some of the pathways for de-excitation to the ground state are illustrated in the simplified Jabłoński-Perrin diagram in Figure 1. The pathways include internal conversion ([math]ic[/math]), decay by fluorescence ([math]kf[/math]), quenching (loss of excitation energy without the emission of light, [math]knf[/math]), or intersystem crossing ([math]isc[/math]) to the triplet state followed by decay by phosphorescence ([math]kpf[/math]).The average time required for a population of fluorophores in the excited state to decay to the ground state is called the fluorescence lifetime, which is described by an exponential function (Figure 1):
[math] I(t)=I_{0}e^{-t/\tau}\tag 1[/math]
where [math]I(t)[/math] is the fluorescence impulse response at time [math]t[/math], [math]I_{0}[/math] is the initial intensity after the excitation pulse, and [math]\tau[/math] is the fluorescence lifetime.