Bring To the Light Art Conservation
Contributions of Photochemistry for a Better Understanding of Cultural Heritage and its Preservation
by Dr. Maria João Melo
Department of Conservation and Restoration, Faculty of Science and Technology - NOVA University of Lisbon, Campus da Caparica, 2829-516 Caparica, Portugal, EU.
1 Preamble
As eloquently summarized by Vincenzo Balzani and Franco Scandola, light is made of photons, and photons are, at the same time, energy quanta and information bits [1]. This duality in the interaction of light with matter is also present in the conservation of cultural heritage. Light as a bit of information provides materials characterization. As a bit, it may reveal a hidden millenary dye or assess the stability of a 20th-century artistic paint. On the other hand, light as energy may be the cause of degradation of the same beautiful dye and of the paint binder, enhancing the decay of many masterpieces worldwide.
2 Studies in ancient natural dyes and color paints
Natural dyes, discovered through the ingenuity and persistence of our ancestors may be found hidden in such diverse places as the roots of a plant, a parasitic insect, and the secretions of a sea snail. In art, natural dyes were used to color a textile or to paint [4].
To be used as a color paint, for example, to create a medieval illumination on a sheet of parchment, colors ours were "tempered" (mixed) with protein binders like glair (egg white), parchment glue (collagen) or even egg yolk [2, 3]. The longevity of a formulation depends not just on the binder, but also on the additives that i) improve the paint application and ii) make it resistant to decay and give it a greater ability to adapt to mechanical stress. The binding medium is the “invisible component” of a paint, but if the binders fail, so will the colors.
2.1 Light as energy quanta: photostability in natural dyes
2.1.1 Indigo as a molecular fossil: color and photophysics
Indigo is one of the most light-stable organic dyes, a characteristic that explains its wide use in antiquity and the pre-modern area[4-6]. The stability of indigo is the reason why it was used in medieval illuminations and by some of the great masters of the 17th and 18th centuries, such as Rubens [7]. The explanation of indigo’s blue color and its derivatives have also been an intriguing and fascinating subject and was explored in detail and ingenuity in the 1970s and 1980s, as described below.
Colour
Some relevant characteristics of indigo are [8-16]:
- i) it's blue colour (with an unusual long wavelength of absorption),
- ii) the effect of substitution on its colour,
- iii) its redox chemistry that interconverts indigo keto form into leuco (reduced) and dehydroindigo (oxidized) species, and
- iv) it's high photochemical stability resulting from a fast excited state proton transfer from the N-H to the C=O group.
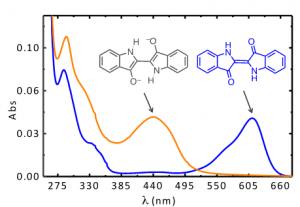
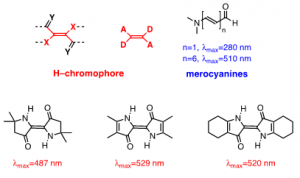
Indigo’s intense blue color is due to its absorption at long wavelengths, an intriguing feature for a relatively small molecule [8-10]. The causes of colour in indigo were investigated in the 1970s with the synthesis of several derivatives - Figure 1[11] - proving that the fundamental chromophore in indigo consists of a central C=C bond (connecting the two rings) together with the nitrogen and carbonyl groups; neither the benzene rings nor the double bonds in the five-member rings are responsible for the characteristic indigo spectrum and blue colour. In summary, resonance is not the cause of indigo’s blue colour[10-14]. Indigo is best described as a donor-acceptor dye, where the amino groups act as the electron donors and the carbonyl groups as the electron acceptors, Figure 2. More recently, DFT calculations supported this description, showing that the HOMO is essentially located in the central C=C bond and the nitrogen atoms and the LUMO in the central C=C bonds and oxygen atoms [15]. The donor groups raise the [math]\pi[/math] orbital of the C=C double bond (HOMO), while the acceptor groups lower the [math]\pi^{*}[/math] orbital (LUMO). In a simplified approximation, the absorption of visible light may be explained by a narrow HOMO-LUMO separation [10].