The Red Edge Effects
Alexander P. Demchenko
Palladin Institute of Biochemistry, Kiev 01030 Ukraine.
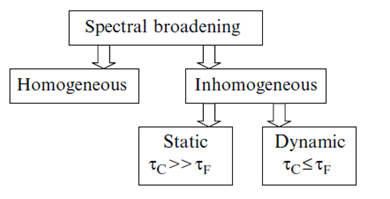
The Red Edge effects are the series of wavelength-selective phenomena that involve the spectral shifts, quenching, anisotropy and lifetimes. Being modulated by the energy of excitation quanta they can be observed both in fluorescence and phosphorescence and produce impact on different excited-state reactions, including the transfer of excitation energy. They are commonly observed in the systems displaying broader distribution of luminophore interaction energy with its environment and in the conditions restricting molecular mobility (polymer matrices, low-temperature glasses, protein molecules, etc.). These effects are consistently explained based on accounting for statistical distribution of fluorescence emitters on their interaction energy with the environment and on the spectral selection of species, the excitation energies of which deviate from mean values. Demonstrating static or dynamic inhomogeneous broading of spectra these phenomena allowed forming a new vision of structural disorder and molecular dynamics in condensed media.
1 Historical Background
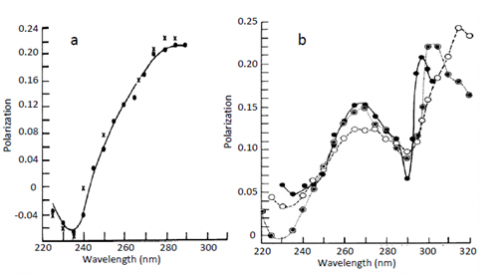
The first observation of Red Edge effects was made by Gregorio Weber [1], see Fig. 1. He demonstrated an almost complete loss of excitation energy transfer evidenced by the loss of depolarization of fluorescence emission when the fluorescence of highly concentrated solutions of tyrosine, tryptophan and their analogs was excited at the long-wavelength edge of absorption spectrum. The conditions for these experiments were the solid fluorophore environments achieved in glass-forming solvents at low temperatures. Later on these studies were extended to other chromophores and other conditions that excluded fluorophore rotation as the mechanism of depolarization, so that in highly concentrated solutions the depolarization should be only due to the excited-state energy homo-transfer (the transfer between the same molecules). Migrating between differently located and oriented fluorophores, the emitted light loses its initial polarization. Meantime in rigid environments at the red edge of excitation band this does not occur and this fact was recognized as a rather general phenomenon (see ref. [2]).
Acheaving its correct interpretation was not easy, since it was in apparent contradiction with paradigm dominated in photophysics that was based on two empirical principles, the Vavilov’s law and the Kasha’s rule. The former postulates independence of emission energy on excitation energy within the absorption band and the latter states that the emission spectrum should occupy the same position on energy scale irrespective of the wavelength of excitation so that the emission must proceed from the lowest electronic and vibrational states. Commonly they were applied considering all chromophores in their ensembles identical on their interaction with their environments. New observations did not break these fundamental principles but introduced new concepts that came with new discoveries. Two grups of Bill Galley [3] in Canada and, independently, Anatoliy Rubinov and Vladimir Tomin [4] in Belarus have reported on finding of a new Red Edge effect – the bathochromic shift of fluorescence spectra at the red edge excitations.
Both of these groups started to consider instead of identical chromophore-environment conditions their distributions on interaction energies. They stated that the spectra of individual fluorophores in solutions shift differently because of photoselection of chromophores differing in these intermolecular interactions. Such distributions result in inhomogeneous broadening of spectra. Within such distribution, the photoselection of the fluorophores, the interactions of which with their environment deviates from their mean values, can be provided at the low-energy slope of excitation band (red edge). These photoselected fluorophores exhibit the wavelength-shifted emission. Of course, the molecular mobility in such systems should be slower than the excited-state lifetime, otherwise the local environments will be mixed and the effect has to disappear.
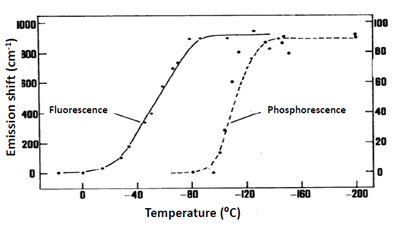
Direct connection between observation of Red Edge effects and the dynamics of solvent molecules can be easily demonstrated (Fig. 2). The wavelength-dependent shift disappears on the transition from cryogenic to room temperatures due to the appearance of molecular motions averaging the chromophore environments. For phosphorescence this transition was found to occur at much lower temperatures than for fluorescence, which correlated with much longer lifetimes providing larger time window for dynamic processes in the solvent. Dependence on the solvent was also remarkable, the effect decreased as one passes from polar to nonpolar vitrified media. These results are quite understandable since the noncovalent dipole-dipole interactions between polar molecules provide the strong contributions to dielectric solvation. With these developments it became clear that all the effects observed on variation of excitation and emission wavelengths should originate not from the violation of fundamental principles, but from their operation in specific conditions, when the ensemble of excited molecules is distributed on interaction energy with molecules in their surrounding.
Detailed studies in the conditions of molecular relaxations fitting the range of lifetimes of fluorescence emission provided not only strong support to the new concepts but allowed observing new phenomena. It was found that the relaxation-induced time-dependent motion of spectra strongly depends on the excitation wavelength. This dependence was specific: the motions of spectra disappear at the red edge, and on shifting the excitation wavelength further to the far anti-Stokes region they can even proceed with the increase of excited-state energy [5, 6]. This phenomenon was called ‘up-relaxation’. For achieving the relaxed state, here instead of releasing the thermal energy the energy is absorbed from the environment, providing the local cooling. It was also found that in inhomogeneously broadened systems the excited-state energy transfer is directed from short-wavelength excited to long-wavelength emitting species resulting in time-dependent motions of spectra. This effect is suppressed at the red-edge excitations.
2 Modern interpretation of Red Edge effects
The optimal conditions in which the Red-Edge effects are observed are now well understood [6, 7]. The dye molecule can absorb only the light quanta that correspond to its electronic transition energy. Being selected from the whole ensemble of chromophores by the energy of electronic transition, this sub-ensemble can possess diverging features observed in fluorescence emission and also different photochemical reactivity. For the observation of strongest effects the dye should be solvatofluorochromic, that is, its fluorescence spectra should respond to the changes in interaction energy with environment by significant shifts. In the case of recording the steady-state spectra the dye environment should be relatively polar but rigid or highly viscous, so that the relaxation times of its dipoles, [math]\tau_{\small R}[/math], should be comparable or longer than the fluorescence lifetime [math]\tau_{\small F}[/math]. When the time-resolved recording is applied, the relaxations should proceed on the same scale as the time scale of emission. Thus, these effects (in fact, their disappearance) are coupled with molecular dynamics in condensed media and allow distinguishing and characterizing rigid, viscous and highly mobile media. The other principle of photoselection is the variation of polarization. Thus, if a dye is excited by polarized light, its emission if it comes from the same oscillator will be also highly polarized. Depolarization occurs only when the time correlation of these selectively excited species is lost due to their rotation or participation in some photophysical process, such as the excitation energy transfer. Operating with excitation light of definite energy and polarization one can excite exclusively those dye molecules, the energy and orientation of electronic transition of which match these excitation parameters.
2.1 Inhomogeneous broadening and the principle of photoselection
When organic dyes are studied in any liquid or solid media, they usually display broad bands in absorption spectra with vibrational structure smoothened or even entirely lost, so that cooling to cryogenic temperatures does not result in improvement of structural resolution. This indicates molecular disorder and means that there exists the so-called inhomogeneous broadening of the spectra [6]. The latter originates from non-equivalence of dye solute environments (sub-states) that results in the distribution of solute-solvent interaction energies. All types of intramolecular and intermolecular relaxations may contribute to the energy difference between the maxima of the absorption and emission spectra, the so-called Stokes shift. The contribution of dielectric relaxations is often the strongest, and the site-photoselection effects can be observed if they are frozen or incomplete. As a result, for every ensemble the electronic transition energies become distributed on the scale of energy and the superposition of spectra belonging to individual chromophores forms an inhomogeneously broadened contour.
In molecular spectroscopy it is a common way to present the electronic transitions generating the absorption and emission spectra as the two-dimensional functions of vibrational and solvation coordinates. Meantime the main difference between these coordinates is the quantized origin of vibrational modes, achieved in a very fast Franck-Condon process. According to Kasha rule, they relax rapidly to the lowest energy level of the first excited state. In contrast, solvation modes are intrinsically over-damped. This allows treating solvation coordinate as a classical coordinate with continuous availability of electronic states. Thus, in molecular ensemble at any finite temperature a Boltzmann distribution in population of different solvent configurations is responsible for the inhomogeneous broadening in the steady state spectra. Thus, the contour of absorption band must contain valuable information on the extent of molecular disorder.
In condensed medium such distributions exist already at the time of excitation. But its manifestation depends on how fast are the transitions between the species forming the excited-state ensemble of states. Depending on these conditions, the broadening of spectra can be either static or dynamic [6]. The signatures of static broadening are observed in rigid environments, when the dynamics described in terms of dipolar relaxation times [math]\tau_{\small R}[/math] is slower than the rate of emission. The broadening is dynamic if the motions in the dye environment occur simultaneously or faster than the emission, [math]\tau_{\small R}\leq \tau_{\small F}[/math]. The static effect that is integrated over the time of emission depends upon the time window. In viscous fluid media (when [math]\tau_{\small R}[/math][math]\approx[/math][math]\tau_{\small F}[/math]) not only the freezing (increasing [math]\tau_{\small R}[/math]) but also the fluorescence quenching (reduction of [math]\tau_{\small F}[/math]) may cause the appearance of Red Edge effects [8]. Therefore, the inhomogeneous broadening effects can also contain the information about the dynamic properties of condensed systems, and the rate of fluorescence emission provides the necessary time scale for these observations (Fig. 3).
Thus, being the major factor that produces broadening of the spectra, inhomogeneous broadening originates from nonequivalence of dye environments in an ensemble of otherwise identical molecules resulting in the distribution on solute-solvent interaction energies [6]. In fact, every molecule is under the influence of different forces produced by configuration of surrounding molecules. Therefore the dye species become distributed on their electronic transition energy and their superposition forms inhomogeneously broadened contour. Because of vibrational contours of electronic absorption bands that extend to higher energies, the possibility of photoselection within the ensemble remains only from the side of low energies of absorption band (red excitation edge).
Excitation at the band edge selects a part of this distribution, the spectroscopic properties of which can be quite different from their mean values. At the long-wavelength edge of absorption band only those species are excited, for which the quanta of absorbed energy are so low that they cannot excite even the 0-0 transition for all members of the ensemble. For those selected excited species the interaction energy with the environment is the strongest and the energy levels occupy the lowest positions, so for them the emission spectrum becomes shifted to longer wavelengths. Thus, the widely explored Red Edge effect is the long-wavelength shift of fluorescence spectra at the red excitation edge. Exciting by monochromatic light and shifting the wavelength from band maximum further and further to the red edge, a smaller and smaller number of dye molecules are excited with correspondent reduction of light emission intensity. Photoselection is also possible from the side of high energy in emission (blue emission edge) because in this case also the broadening of purely electronic (0-0) transition can be detected being not spoiled by vibronic contributions [7].
It has become evident that the Red Edge effects do not break the Kasha rule. The Kasha rule must be applied not to whole ensemble but to individual emitters forming their inhomogeneously broadened ensemble.
2.2 Connection with molecular relaxations
The time window for observing these relaxations in fluorescence spectra is determined by the rate of fluorescence emission. Depending on molecular mobility in the medium the process of attaining a new equilibrium (relaxation) may be faster, slower or occur simultaneously with the emission decay. In the case if it occurs simultaneously with the decay, complex emission-wavelength dependence should be observed for the decay kinetics. Due to temporal decrease of excitation energy the spectra move to lower energies until the relaxed state is reached. Determination of relaxation times in these cases is still complicated because the spectral information in limiting cases of low and high relaxation dynamics is needed. The case of low spectral dynamics can be achieved by decreasing the temperature (freezing the relaxations) or by introduction of dynamic quenchers (decreasing the lifetime). However, the limit of high spectral dynamics is extremely difficult to reach without the risk of damaging the studied structures.
Introducing a new dimension, the Red Edge effects offer a solution of this problem [9, 10]. When the relaxation is complete, it produces new dynamic distribution of interacting species, ‘mixing’ different environments. Being selected at any wavelength, the sub-population of dyes is rapidly mixed within the whole population, so that the spectra become independent on excitation wavelength ([math]\lambda _{ex}[/math]) and the Red Edge effect disappears. Thus, the dynamic information about molecular relaxations can be obtained in simple steady-state measurements using [math]\tau_{\small F}[/math] as a time marker and analyzing the Red Edge effects.
One of the most intriguing properties of structurally disordered materials (liquid or solid) is the huge dispersion of structural relaxation rates; therefore a simple model operating with single [math]\tau_{\small R}[/math] and [math]\tau_{\small F}[/math] values may not be applicable in all cases. However, being conceptually correct, it helps to understand the basis of studied phenomena and the interpretation of many experimental data on quantitative level is quite satisfactory.
The new view on inhomogeneous distributions of light emitters in molecular ensembles required re-interpretation of many time-resolved spectroscopic data. Commonly, the spectroscopic observations of dielectric (dipolar) relaxation are provided by excitation at the band maximum and the recording the shifts of emission spectra to longer wavelengths as a function of time. As a result, when observed at the blue edge, the emission decay contains short-decaying positive component(s) due to fast temporal decrease of a number of emitters possessing higher energies. When observed at the red emission edge the decay contains a negative component due to increase with time of the number of excited-state species emitting at longer wavelengths. The non-exponential decay functions recorded at different emission wavelengths are used for constructing the time-resolved spectra. The Red Edge effects introduce new dimension into this picture and allows probing the redistribution of species with different emission energy as a function of time between the sites in an ensemble [5]. Moreover, these studies bring in new concept of relaxation, which is the re-organization in ensemble of distributed states on the achievement of excited-state equilibrium [6]. Whereas the major part of the excited dye population demonstrates the motion of spectra to longer wavelengths, the spectra of the sub-population selected at the red edge may not move. This is because those species are photoselected, the interactions of which with the environment are already strong, close to the relaxed state. When the dye is excited by energy lower than that of the relaxed state, the spectra move to opposite direction, to higher energies (up-relaxation) [5]. The emission kinetics excited at the red edge becomes uni-modal and almost single-exponential. The spectra are initially more narrow (since a part of the distribution is selected) and are broadened in the course of relaxation due to redistribution to a broader number of different sites. If fluorescence is selectively excited by a narrow-band pulse, then a time-dependent broadening (spectral diffusion) is observed, and due to temporal depopulation of ‘selected’ fluorophores, a selective decrease of [math]\tau_{\small F}[/math] is observed at the frequency of excitation. Site-photoselection at the red edge results in disappearance of these effects.
Thus, the time-resolved fluorescence methods can be easily extended to experiments with site-selective excitation. These results allowed achieving better understanding the molecular relaxation phenomena introducing a new very productive concept: the light absorption and emission spectra being formed by inhomogeneous ensemble of molecules reflect the stochastic dynamics of their formation and re-arrangement in time. Therefore the possibility appears to extract dynamic information by comparing the data of spectroscopic, time-resolved and anisotropy studies obtained for the whole ensemble on comparison with its selected part. The relaxation in molecular ensemble is not only the change of average energy of excited fluorophores (commonly, it decreases), it is also the re-arrangement of initially selected species, their mixing within the whole ensemble.
3 Diversity of wavelength-selective effects
Manipulating with excitation wavelength the following effects can be observed: - Variation of temperature. Both [math]\tau_{\small R}[/math] and [math]\tau_{\small F}[/math] depend on temperature, so the correlation between them, the function [math]\frac{\tau_{\small F}}{\left ( \tau_{\small F}+\tau_{\small R} \right )}[/math], should be temperature-dependent. Usually the relaxation rate increases with temperature faster than the fluorescence rate. In some cases structural changes in the system with the change of [math]\tau_{\small R}[/math] can be detected, but there may be the cases when the relaxation rates change without conformational change.
- The effects of fluorescence quenchers. The result of collisional quenching is the change of fluorescence lifetime [math]\tau_{\small F}[/math] that shortens the time window for relaxations and increases the Red Edge effects.
- The time-resolved observations. The spectra move to longer wavelengths as a result of dielectric relaxation with the decrease of energy (the common case). They stop to move if by shifting the wavelength the isorelaxation point is achieved, and they start to move to shorter wavelengths at the far red edge, when the relaxed state is of higher energy (the case of “up-relaxation”) [5].
Photoselection can be observed also in emission spectra. This is possible only at the blue (short-wavelength) edge of emission band, i.e. at the high energy side of [math]0-0[/math] transition in emission [11]. As a result, the excitation spectrum will gradually shift to the blue at the ‘blue edge’ of emission band. Thus, in emission spectrum the site-selectivity leads to another site-selective effect – the dependence of excitation spectra on emission wavelength. Similarly to the other Red-Edge effects, this effect disappears as a result of relaxation.
Different Red Edge effects can be observed on excitation with polarized light, indicating that fluorophore rotations can be site-selective. Such effects may be produced due to variation of time window, in which fluorophore rotations are observed. Excitation at the red edge suppresses the relaxational shift of spectra and makes the emission decay more homogeneous [12].
4 Photochemical reactions modulated by the Red Edge effects
The excited-state reactions, the rate of which depends on the energy and dynamics of weak noncovalent interactions with the environment can be modulated by site-selection effects. The study of this coupling with molecular relaxations can be used as an important clue for elucidating their mechanisms. In unrelaxed states the distribution of excited-state species on their interaction energies with the environment may result in distributed reaction kinetics [13]. It was shown that the part of this distribution that interacts stronger with the environment may exhibit an extreme increase in reactivity in intramolecular electron transfer reaction and a decreased reactivity in proton transfer [14] and energy transfer reactions [15, 16]. Site-photoselective spectroscopy allows not only to characterize the selective photochemical reactivity but also to provide the means to model the reactions occurring in the ground states, especially those of them which possess low intrinsic activational barriers and depend on dynamics in the environment. Those are many biocatalytic reactions.
5 Directional excited-state energy transfer and Red Edge effects
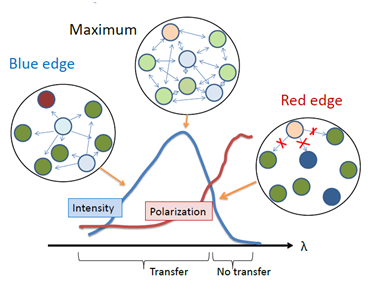
The failure of energy homo-transfer between the dyes in highly concentrated solutions in rigid and highly viscous media (see Fig. 1) can be naturally explained by site-photoselection within the inhomogeneously contour of absorption band. Here the same molecules serve the role of both donors and acceptors but being located in variable environments they display non-identical absorption and emission spectra. The species from upper part of the distribution in excited-state energy can transfer their energy to another species on the same or lower energy level. In contrast, the species from the lower part of the distribution (their effective concentration is low) can transfer their energy only to other species of the lower part of this distribution. Because of small number of these selected species, such transfer is a low probable event. Thus, due to the presence of inhomogeneous broadening, the excited-state energy transfer between chemically identical molecules is not random, it is directed from those members of the ensemble which emit at shorter wavelengths to those which absorb at longer wavelengths [17]. The shift of spectra due to directed transfer can be observed as a function of time, even if the environment is completely immobile [nasza rabota]. There is also an emission analog of this effect: in concentrated solid dye solutions the energy homo-transfer fails to occur at the short-wavelength edge of emission band. In this case the emission of the dyes serving as acceptors and emitting at lower energies is not recorded, and the emission of donors remains at high energies and is highly polarized (Fig. 4).
Suppressesing the energy transfer, transition to the red-edge of excitation allows introducing an important “no-transfer” reference limit. At these wavelengths the fluorophores emit individually, their emission spectra are similar to that observed at low concentrations (at the same excitation) and the decay kinetics is simplified.
6 Red Edge effects and ground-state heterogeneity
In different structurally heterogeneous systems the excitation wavelength dependence can appear due to factors unrelated to Red Edge effects. There may be different types of impurities as well as ground-state heterogeneity in dye state (ionization, charge-transfer complexes, H-bonding). Operating on atomic scale of distances, the positional heterogeneity can arise from imprecise location and orientation of the dye, e.g. its distribution between the sites of different polarities or at the surface of molecule or nanoparticle. Apparent spectral broadening can be the result of all these factors, and this could result in preferable excitation of one of the species with respect to others, resulting in shifted fluorescence spectra. In our work [18] we tried to introduce the criteria that distinguish the effects of inhomogeneous broadening and those that originate from positional (ground-state) heterogeneity. When the excitation wavelength dependencies of fluorescence spectra are analyzed on a broad scale, the Red Edge effects demonstrate a very characteristic shape with the absence of the shifts of fluorescence spectra as a function of excitation wavelength at the maxima and short-wavelength wing of excitation bands but an unlimited increase of effect at the red excitation edge. In contrast, the ground-state heterogeneity comes from superposition of absorption (excitation) spectra of the dyes present in different forms (e.g. rotational isomers) or residing in different locations. Because the quantum yield, anisotropy and lifetime of a dye in these forms or locations can differ, the shapes of measured excitation-wavelength dependences as a general case is variable, and even may become sigmoid. The study of polarization and emission decay kinetics as a function of excitation wavelength may also serve a good criterion.
7 Connection with the studies of single molecules
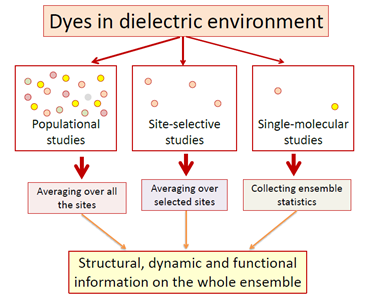
It is common for us to assume that molecules of the same chemical composition must have identical properties. In reality though, every molecule in a condensed medium is different. Unlike ideal crystal, where only vibrational motions are allowed, real condensed matter systems display much greater possibilities for intermolecular configurations resulting in structural, energetic and dynamic molecular disorders. The power of Red Edge effects in these studies is in ability to compare the static and dynamic spectroscopic information of the whole ensemble of innumerable number of molecules with its sub-ensemble of energy-selected species. Further decrease of this ensemble leads us to a single molecular level. This allows removing the usual ensemble-averaged picture and obtaining the most valuable information on the intensity, excitation and emission spectra, polarization, fluorescence decay rate of chosen individual molecules and of their chemical and photochemical reactivity. In the studies of single molecules the concept on broad distribution of solvation energies in ensembles of fluorophore molecules in condensed media has got final confirmation.
The single-molecule detection allows providing a conceptually important move from ensemble analysis to individual event analysis. Individual molecules can be fitted to statistical ensemble. The more informative histograms based on single molecular studies can be recorded, in which the responses from the members of molecular ensemble are seen as distinct events [19]. Thus, the role of Red Edge effects in photophysical studies of dyes in condensed phase in comparison with common spectroscopic experiments and investigations of single molecules can be summarized as follows (see Fig. 5):
- The standard spectroscopic measurements yield the information on ensemble of emitters. Those are the average values of studied parameters for a large number of molecules. These studies remain to be of great value, since in real systems the physical behavior and chemical reactivity is the property of whole molecular ensembles.
- The Red-Edge effects allow the observations of inhomogeneous broadening and molecular dynamics based on comparison in behavior of molecular ensemble and its selected sub-ensemble. They have become ‘classical’, playing their important role as a part of standard spectroscopic techniques.
- The level of single molecules allows studying the fluctuations and statistical distributions, and an inhomogeneous contour of the ensemble of molecules can be built from these individual contributions. By removing completely the ensemble averaging, the distribution of every measured parameter can be obtained and a frequency histogram of the actual distribution of values can be constructed. Such distribution contains more information than the average value alone [20].
8 Summary
The observation and analysis of Red Edge effects has developed into powerful methodology for studying molecular disorder and its coupling with molecular dynamics. With years of exploration, the variation of excitation wavelength has become an important spectroscopic tool generating informative response in all optical parameters: spectral shifts, quenching, anisotropy and lifetimes. This methodology is simple in application; it can easily complement other fluorescence spectroscopic methods in studying microscopically heterogeneous systems of different kind, including biological macromolecules in solutions and complex nanoscale systems. The numerous obtained results were summarized in different reviews demonstrating successful applications in the studies of proteins, biomembranes, polymers, ionic liquids, etc. The demonstraton that proteins in solutions on the time scale of nanoseconds behave as nanoscopic solids with the dynamics slower by several orders of magnitude than the surrounding solvent [10, 21] has become a fundamental knowledge. The great number of recently published articles indicates that this field is blooming, and many more advancements are to be expected.
One of these new fields is the site-selective photochemistry. In different types of photochemical reaction dynamics (intramolecular charge transfer, electron and proton transfer, excitation energy transfer) direct connection was established of the reaction yield and rate with the interaction of reaction site with its environment and the dynamics of this environment. The site-selective inhomogeneous behaviour was demonstrated. Different nanoscale compositions can be designed to provide and control these reactions, including light harvesting and wavelength conversion with the possibility to modulate them at the red excitation edge.
Finally, we recognize that molecular disorder is not a complication in spectroscopic studies if we know how to extract extremely important information regarding the behaviour of condensed matter on molecular scale and on the scale of intermolecular interactions. This information is especially valuable if the observed systems are microscopically heterogeneous and exhibit hierarchical dynamic features.
9 References
[1] G. Weber, "Fluorescence-polarization spectrum and electronic-energy transfer in tyrosine, tryptophan and related compounds," Biochemical Journal, vol. 75, p. 335, 1960.
[2] A. P. Demchenko, "Weber’s Red-Edge Effect that Changed the Paradigm in Photophysics and Photochemistry," Perspectives on Fluorescence: A Tribute to Gregorio Weber, pp. 95-141, 2016.
[3] W. C. Galley and R. M. Purkey, "Role of heterogeneity of the solvation site in electronic spectra in solution," Proceedings of the National Academy of Sciences, vol. 67, pp. 1116-1121, 1970.
[4] A. Rubinov and V. Tomin, "Bathochromic luminescence in solutions of organic dyes at low temperatures," Opt. Spektrosk., vol. 29, pp. 578-&, 1970.
[5] N. Nemkovich, et al., "Intermolecular up-relaxation in phthalimide solutions at excitation by frequency tuned dye laser," Opt. Spektrosk, vol. 49, pp. 274-283, 1980.
[6] N. A. Nemkovich, et al., "Inhomogeneous broadening of electronic spectra of dye molecules in solutions," in Topics in fluorescence spectroscopy. vol. 2, J. R. Lakowicz, Ed., ed New York: Plenum Press, 1991, pp. 367-428.
[7] A. P. Demchenko, "The red-edge effects: 30 years of exploration," Luminescence, vol. 17, pp. 19-42, Jan-Feb 2002.
[8] V. Tomin, et al., "Effect of quenchers on fluorescence spectra of dye polar solutions," Optika Spektrosk, vol. 34, pp. 1108-1111, 1973.
[9] A. P. Demchenko and A. S. Ladokhin, "Red-Edge-Excitation Fluorescence Spectroscopy of Indole and Tryptophan," European Biophysics Journal with Biophysics Letters, vol. 15, pp. 369-379, 1988.
[10] A. Demchenko, Ultraviolet spectroscopy of proteins. Heidelberg: Springer Verlag, 1986.
[11] V. Pavlovich, "Dependence of the spectra of excitation of dipole molecule solutions on the recording wavelength," Journal of Applied Spectroscopy, vol. 25, pp. 1141-1147, 1976.
[12] D. M. Gakamsky, et al., "Fluorescence Decay Time Distribution for Polar Dye Solutions with Time-Dependent Fluorescent Shift," Biophysical Chemistry, vol. 44, pp. 47-60, Aug 1992.
[13] A. P. Demchenko, "Does biocatalysis involve inhomogeneous kinetics?," FEBS letters, vol. 310, pp. 211-215, 1992.
[14] V. Tomin and R. Jaworski, "Modulation of the proton transfer rate by excitation photons," Optics and spectroscopy, vol. 114, pp. 729-736, 2013.
[15] A. P. Demchenko and A. I. Sytnik, "Site-selectivity in excited-state reactions in solutions.," J. Phys. Chem., vol. 95, pp. 10518-10524., 1991.
[16] A. P. Demchenko and A. I. Sytnik, "Solvent reorganizational red-edge effect in intramolecular electron transfer," Proc Natl Acad Sci U S A, vol. 88, pp. 9311-4, Oct 15 1991.
[17] I. Gulis and A. Komyak, "Peculiarities of inductive-resonance energy transfer in the conditions of organic molecule electronic levels inhomogeneous broadening," Zh. Prikl. Spektrosk, vol. 27, pp. 841-845, 1977.
[18] A. P. Demchenko, "Site-selective Red-Edge effects. Chapter 4.," Methods in Enzymology, vol. 450, pp. 59-78, 2008.
[19] A. A. Deniz, et al., "Single-molecule biophysics: at the interface of biology, physics and chemistry," Journal of the Royal Society Interface, vol. 5, pp. 15-45, Jan 2008.
[20] J. Hohlbein, et al., "Surfing on a new wave of single-molecule fluorescence methods," Physical biology, vol. 7, p. 031001, 2010.
[21] A. P. Demchenko, " Red-edge-excitation fluorescence spectroscopy of single-tryptophan proteins," Eur. Biophys. J., vol. 16, pp. 121-9, 1988.