Photostable Compounds
Dr. Susana Encinas
Departamento de Química, Universitat Politècnica de València, Camino de Vera s/n, 46022 Valencia, Spain.
Contents
Light can change the properties of different materials and products. This is often observed as bleaching of coloured compounds like paint and textiles. Photostability has for many years been a main concern within several fields of industry, e.g. the textile, paint, food, cosmetic and agricultural industries. In the field of pharmacy, the number of drugs found to be photochemically unstable is steadily increasing. In this context, the term photostability is used to describe how a compound responds to light exposure and includes not only degradation reactions but also other processes such as formation of radicals, energy transfer and luminescence.
Each organism is a product of its response to the pressures of the environment, in order to establish a balance between the organism and its environment. This balance causes changes in the subject, which can be harmful or beneficial to their existence. Therefore, the possibility that several compounds in combination with sunlight may be beneficial or harmful to the patient should be considered.
1 Photophysical and Photochemical Aspects of Photostability
The fact that a substance absorbs radiation in the ultraviolet or visible region of the electromagnetic spectrum means that it is absorbing energy that is sufficient to break a bond in the molecule. Thus the property of absorption is a first indication that the substance may be capable of participating in a photochemical process leading to its own decomposition. According to the Grotthus’ law of photochemistry, no photochemical (or subsequent photobiological) reaction can occur unless electromagnetic radiation is absorbed. The absorption spectrum of a compound is therefore an immediate way of determining the wavelength range to which the substance may be sensitive.
There are two important factors to ponder in relation to the potential of a compound to be degraded following absorption of electromagnetic radiation. First, the absorption spectrum is normally described by the maximum absorption wavelength and the molar absorptivity at that wavelength, but the spectrum can be broad and any overlap of the absorption spectrum with the output of the photon source impinging upon it has the potential to lead to photochemical change. Second, the decomposition may be initiated by another close compound (for example, in a drug formulation) that has the absorption characteristics that overlap with the incident radiation while the main compound does not. This process is the photosensitization and the absorbing compound is the photosensitizer. [1] (see Photosensitization entry [[1]]).
Photochemical damage to a substance is initiated by the absorption of energy by the compound itself of by a photosensitizer. Many photochemical reactions are complex, and may involve a series of competing for reaction pathways in which oxygen may play a significant role. In fact, the great majority of photoreactions in biological systems involve the consumption of molecular oxygen and are photosensitized oxidation processes.[2]
The photophysical processes are usually well described by the Jablonsky diagram (see Photosensitization entry) and they could lead to a wide photochemical reactivity. Among the most common reaction types that a compound might experience under photon absorption are addition, cyclization, N-dealkylation, decarbonylation, decarboxylation, dehalogenation, dimerization, oxidation, reduction, isomerization, rearrangement, and/or hydrolysis [3].
Furthermore, the concept of photostability covers a very wide field and it could be applied to many issues such as drugs, dyes, labels, solar filters, etc. Next, a few explicative examples like drugs and solar filters are presented.
2 Drugs Photostability
Drugs are agents designed to produce a specific and beneficial effect in the body, but they can lead to adverse reactions, of very different nature, which are assumed in terms of the risk-benefit ratio of their use. In general, precautions are taken to prevent photodegradation of drugs during storage, but changes induced by light in a patient who has been treated with a drug can lead to significant side effects. Within the adverse reactions caused by drugs is the photosensitization, i.e. the drug cause light-induced side effects after administration to the patient by interaction with endogenous substances [1] (see Photosensitization entry[[2]]).
Until now, most reports on the biological activity of drugs come from dermatologists, and show adverse effects such as erythema, edemas, followed by hyperpigmentation and desquamation. Although drugs are the major cause of skin photosensitization, the incidence of the photosensitivity they cause is not easy to know, since there is a great variability, probably due to local variations in skin type, light exposure and drug doses [4].
In this sense, there is great diversity at the level of therapeutic groups, structural formula, etc., which makes it difficult to predict a priori the same photobiological activity. The difficulty of establishing a satisfactory structure-activity relationship is evident. Agents acting as photoallergic agents, as well as those that cause contact dermatitis, are usually liposoluble and with a low molecular weight. Like phototoxic agents, they tend to have conjugated structures capable of absorbing light radiation [5].
Therefore, it is important to know the photobiological activity of drugs because a) allowsvarying the molecular structure to minimize the side effects conserving the desired pharmacological effects, and b) allowstaking advantage of the light interaction with the biological processes for therapeutical purposes.
3 Solar Filters Photostability
Nowadays, there is an increasing need for good topical sunscreens to prevent the well-documented damaging effects of ultraviolet (UV) light on human skin (see Photoprotection entry). The ideal sunscreen should be such that no photochemical or photosensitizing transformation of its components occurs within the formulation or on the skin. Photochemical stability is indeed the most important characteristic of an effective UV filter since the light-induced decomposition of the sunscreen agent not only reduces its photoprotective power but can also promote phototoxic or photoallergic contact dermatitis.[6]
Nevertheless, several compounds used as UV-filters exhibit some photoreactivity leading to the formation of photoproducts that can absorb in different spectral regions, thus reducing their photoprotective efficacy. Moreover, UV filters can also display some photosensitizing effects: their photochemical intermediates or stable photoproducts can interact with skin components, mediating phototoxic and/or photoallergic processes (see Photosensitization entry [[3]]). Consequently, as new compounds of unknown toxicology can be formed, evaluation of potential hazards associated with the use of a sunscreen requires a detailed study of its photochemistry.
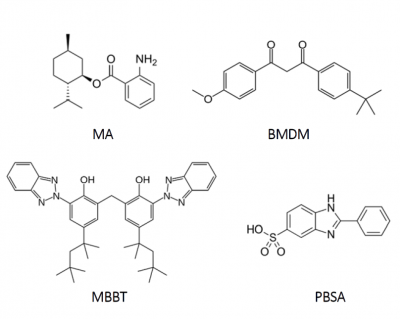
In this context, the UV absorbers used in sunscreens (Figure 1) must be photostable under the conditions of use. Among the most commonly used compoundspara-aminobenzoic acid (PABA) was patented in 1943 with the water resistant ability. However, it can elicit photocontact allergies and possibly autoimmune diseases. Moreover, its decomposition is able to produce potentially carcinogenic products. In the 1980s, benzophenone-3 (BP3) became the most frequently used component of sunscreen formulations and it also reported acute toxic effects.[7]
On the other hand, the principle of a fast internal conversion is realized, for instance, in the menthyl anthranilate (MA) filter because of the orthoamino group, resulting in excellent photostability. In others, this is realized via an orthohydroxy group forming hydrogen bonds (e.g. bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT) and methylene bis-benzotriazolyl tetramethylbutylphenol (MBBT)). In fact, UVA filters that fulfil both efficacy and photostability requirements are rare. For instance, benzophenones provide broad-spectrum UVB and UVA protection; however, they are photolabile and their oxidation can interrupt the antioxidant system. Also butyl methoxydibenzoylmethane (BMDM), a potent UVA filter, undergoes rapid photodegradation. [8]
Furthermore, there are studies demonstrating that some filters react under UV irradiation as it is the case of phenylbenzimidazole sulfonic acid (PBSA) that generates a variety of free radicals and active oxygen species that may be involved in the deoxyribonucleic acid (DNA) photodamage. It would appear that PBSA has the potential to act as a photosensitizer and interact with DNA, even as it protects skin cells from the effects of direct sunlight.[9]
On the whole, the faster the rate of internal conversion, the better is the photostability of an absorber molecule. If the excitation energy cannot be disposed off by energy transfer, by emission of light or if the absorbed energy is not sufficiently and speedily dissipated into heat, chemical bonds of the UV absorber molecule may break or rearrange, resulting in degradation of the UV filter.In addition, sunscreens must dissipate the absorbed energy efficiently through photophysical and photochemical pathways that rule out the formation of singlet oxygen, other reactive oxygen species (ROS), and other harmful reactive intermediates.[10, 11]
3.1 Photostabilization by Triplet Quenching: Triazines
Considering a photoprotective strategy (see Photoprotection entry [[4]]), sunscreen formulations including triplet quenchers could provide effective protection from the potential phototoxic and photoallergic effects derived from poor photostability of some filters. [12] Then, photounstable UV absorbers may be additionally stabilized by employing triplet quenchers. In this sense, being aware of these mechanisms and applying them for specific UV filter combinations can help in designing efficient sunscreens.
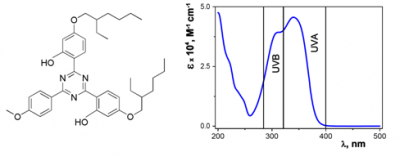
Among the most common UV light filters, BEMT is reported as highly photostable, with a recovery higher than 99% after irradiation being a broad-spectrum (280-380 nm) agent (Figure 2 right). It is oil soluble and has been used successfully to improve the photostability and efficacy of sunscreens containing BMDM and ethylhexyl methoxycinnamate (EHMC). [13] Moreover, it has been evidenced that BEMT may act as a triplet quencher and can be used to stabilize photounstable filters. Because of its relatively large size, BEMT filter rarely cause allergic contact dermatitis, systemic absorption, or endocrine-like effects.[6]
Given its molecular symmetry (Figure 2 left), the presence of electron-releasing groups such as hydroxyl group substituted on the aromatic rings provides to BEMT an optimal structure for energy dissipative processes allowing electron resonance delocalization upon absorption of a photon, and is most probably able to deactivate sensitizers trough energy transfer (triplet-triplet energy transfer) leading to the isomerization of the acceptor (reversible photoisomerization and deactivating capacity). [10] To return to its ground state it can efficiently dissipate the accepted energy through intramolecular hydrogen transfer in the excited state followed by internal conversion and thermal deactivation.[14]
In this context, a new related UV filter, known as tris-biphenyl triazine, has just been launched on the market. Its broad spectrum associated with its qualities in terms of efficacy and photostability make it a choice ingredient for the formulation of sun protection products.[15]
4 References
[1] Tonnesen, H. H., Ed. (1996) Photostability of drugs and drug formulations. Taylor & Francis Ltd.
[2] Spikes, J. D. (1989) The science of photobiology. K. C. Smith, Ed. Plenum Press: New York, 3:79-110.
[3] Greenhill, J. V.; McLelland, M. A. (1990) Progr. Med. Chem. 27:51-121.
[4] Johnson, B. E.; Ferguson, J. (1990) Seminars in Dermatology 9:39-46.
[5] Miranda, M. A. (1997) In vitro methods in pharmaceutical research. J. V. Castell, Ed. Academic Press: London, 13:289-315.
[6] Herzog, B.; Wehrle, M.; Quass, K. (2009) Photostability of UV absorber systems in sunscreens. Photochem. Photobiol. 85:869-878.
[7] Maier, T.; Korting, H. C. (2005) Sunscreens-Which and what for? Skin Pharmacol. Physiol. 18:253-262.
[8] Jansen, R.; Osterwalder, U.; Wang, S. Q.; Burnett, M.; Lim, H. W. (2013) Photoprotection Part II. Sunscreen: Development, efficacy and controversies. J. Am. Acad. Dermatol. 69:1-14.
[9] Inbaraj, J. J.; Bilski, P.; Chignell, C.F. (2002) Photophysical and photochemical studies of 2-phenylbenzimidazole and UVB sunscreen 2-phenylbenzimidazole-5-sulfonic acid. Photochem. Photobiol. 75:107-116.
[10] Chatelain, E.; Gabard, B. (2001) Photostabilization of butyl methoxydibenzoylmethane (Avobenzone) and ethylhexyl methoxycinnamate by bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a new UV broadband filter. Photochem. Photobiol. 74:401-406.
[11] Serpone, N.; Dondi, D.; Albini, A. (2007) Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg. Chim. Acta 360:794-802.
[12] Paris, C.; Lhiaubet-Vallet, V.; Jimenez, O.; Trullas, C.; Miranda, M. A. (2009) A blocked diketo form of avobenzone: Photostability, photosensitizing properties and triplet quenching by a triazine-derived UVB-filter. Photochem. Photobiol. 85:178-184.
[13] Lhiaubet-Vallet, V.; Marin, M.; Jimenez, O.; Gorchs, O.; Trullas, C.; Miranda, M.A. (2010) Filter-filter interactions. Photostabilization, triplet quenching and reactivity with singlet oxygen. Photochem. Photobiol. Sci. 9:552-558.
[14] McGarry, P. F.; Jockusch, S.; Fujiwara, Y.; Kaprinidis, N. A.; Turro, N. J. (1997) DMSO solvent induced photochemistry in highly photostable compounds. The role of intermolecular hydrogen bonding. J. Phys. Chem. A 101:764–767.
[15] Couteau, C.; Paparis, E.; Chauvet, C.; Coiffard, L. (2015) Tris-biphenyl triazine, a new ultraviolet filter studied in terms of photoprotective efficacy. Int. J. Pharm. 487:120-123.