Bring To the Light Art Conservation
Contributions of Photochemistry for a Better Understanding of Cultural Heritage and its Preservation
by Dr. Maria João Melo[[1]]
Department of Conservation and Restoration, Requimte, Faculty of Science and Technology - NOVA University of Lisbon, Campus da Caparica, 2829-516 Caparica, Portugal, EU.
Contents
- 1 Preamble
- 2 Studies in ancient natural dyes and color paints
- 3 The invisible in paint: the binding medium
- 4 Box 1: Measuring a reaction quantum yield in a transparent gel
- 5 Box 2: Measuring a reaction quantum yield ([math]\Phi_{R}[/math]) in a solid polymer matrix
- 6 Box 3: Norrish reaction mechanisms in PVAc photodegradation
- 7 References
1 Preamble
As eloquently summarized by Vincenzo Balzani and Franco Scandola, light is made of photons, and photons are, at the same time, energy quanta and information bits [1]. This duality in the interaction of light with matter is also present in the conservation of cultural heritage. Light as a bit of information provides materials characterization. As a bit, it may reveal a hidden millenary dye or assess the stability of a 20th-century artistic paint. On the other hand, light as energy may be the cause of degradation of the same beautiful dye and of the paint binder, enhancing the decay of many masterpieces worldwide.
2 Studies in ancient natural dyes and color paints
Natural dyes, discovered through the ingenuity and persistence of our ancestors may be found hidden in such diverse places as the roots of a plant, a parasitic insect, and the secretions of a sea snail. In art, natural dyes were used to color a textile or to paint [2-4].
To be used as a color paint, for example, to create a medieval illumination on a sheet of parchment, colors were "tempered" (mixed) with protein binders like glair (egg white), parchment glue (collagen) or even egg yolk [2, 3]. The longevity of a formulation depends not just on the binder, but also on the additives that improve the paint application and make it resistant to decay and give it a greater ability to adapt to mechanical stress. The binding medium is the “invisible component” of a paint, but if the binders fail, so will the colors.
2.1 Light as energy quanta: photostability in natural dyes
2.1.1 Indigo as a molecular fossil: color and photophysics
Indigo is one of the most light-stable organic dyes, a characteristic that explains its wide use in antiquity and the pre-modern era [4-6]. The stability of indigo is the reason why it was used in medieval illuminations and by some of the great masters of the 17th and 18th centuries, such as Rubens [7]. The explanation of indigo’s blue color and its derivatives has also been an intriguing and fascinating subject and was explored in detail and ingenuity in the 1970s and 1980s, as described below.
Colour
Some relevant characteristics of indigo are [8-16]:
- i) it's blue colour (with an unusual long wavelength of absorption),
- ii) the effect of substitution on its colour,
- iii) its redox chemistry that interconverts indigo keto form into leuco (reduced) and dehydroindigo (oxidized) species, and
- iv) it's high photochemical stability resulting from a fast excited state proton transfer from the N-H to the C=O group.
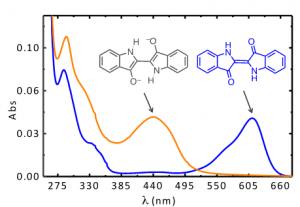
Indigo’s intense blue color is due to its absorption at long wavelengths, an intriguing feature for a relatively small molecule [8-10]. The causes of colour in indigo were investigated in the 1970s with the synthesis of several derivatives - Figure 1 [11] - proving that the fundamental chromophore in indigo consists of a central C=C bond (connecting the two rings) together with the nitrogen and carbonyl groups; neither the benzene rings nor the double bonds in the five-member rings are responsible for the characteristic indigo spectrum and blue colour. In summary, resonance is not the cause of indigo’s blue colour [10-14]. Indigo is best described as a donor-acceptor dye, where the amino groups act as the electron donors and the carbonyl groups as the electron acceptors, Figure 2. More recently, DFT calculations supported this description, showing that the HOMO is essentially located in the central C=C bond and the nitrogen atoms and the LUMO in the central C=C bonds and oxygen atoms [15]. The donor groups raise the [math]\pi[/math] orbital of the C=C double bond (HOMO), while the acceptor groups lower the [math]\pi^{*}[/math] orbital (LUMO). In a simplified approximation, the absorption of visible light may be explained by a narrow HOMO-LUMO separation [10].
Moreover, indigo shows a very efficient aggregation mechanism in solution with an equilibrium constant for the dimer formation of about 1x10[math]^{4}[/math]dm[math]^{3}[/math]mol[math]^{-1}[/math] at 298 K [16] and, by means of theoretical calculations, it was demonstrated that stable dimer and trimer aggregates in vacuo and in solution can be formed [17]. Both experimental and theoretical results shown a batochromic shift of the lowest electronic transition responsible for the color and this behavior, due to the intermolecular hydrogen bond, justify the broadening to the red of the spectrum of indigo on solid state and pictorial layers.
Photoprotection by excited proton transfer.
Indigo has a fluorescence quantum yield of [math]\Phi_{F}[/math]= 0.0023, a fluorescence lifetime [math]\tau[/math]= 0.14 ns, and an energy for the first lying singlet excited state of 15728 cm[math]^{-1}[/math][5, 16, 18]. With such a weak emitter, it was a challenge to obtain the yields (and rate constants) of the radiationless deactivation channels (internal conversion, [math]\Phi _{IC}[/math] and singlet to triplet intersystem crossing, [math]\Phi _{ISC}[/math]). This was done by obtaining the triplet energy with pulse radiolysis energy transfer experiments (ET= 1.0 ± 0.1 eV), followed by a determination of the [math]\Phi _{ISC}[/math] (=0.0066) from photoacoustic calorimetry [19]. These numbers show that over 99.99% of the quanta are lost through non-radiative channels.
In theory, the efficiency of the internal conversion deactivation channels may result from a proton transfer in the excited state (ESPT) or be the consequence of the golden rule for radiationless transitions: when the energy difference between the ground and first singlet ([math]\Delta E=S_{1}-S_{o}[/math]) is very small, which is the case of indigo, it favours an efficient coupling between the [math]S_{0}[/math] and [math]S_{1}[/math] vibrational modes [5, 16].
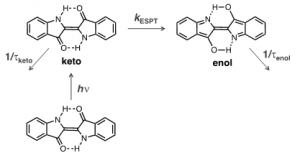
It was demonstrated in both experimental [19-21] and theoretical calculations [15] that the high stability of indigo has its mechanism associated with a fast proton transfer in the excited state (ESPT), although there is still a dispute about whether the excited proton transfer involves a concerted proton transfer between the two N-H and two C=O groups or from a single group, and if it is intra or intermolecular. This ESPT is schematically depicted in Figure 3: the keto excited species is converted into its enol isomer with a rate constant of ~8.4×10[math]^{10}[/math] s[math]^{-1}[/math], in which the existence of these two excited species is in agreement with the biexponential decay observed in indigo [20].
Considering that in the solid state, the hydrogen-bond network connecting several indigo molecules, may lead to an enhancement of the non-radiative pathway efficiency; we may predict, on one side, an increase on its photostability in the solid state, while, the already low fluorescence quantum yield becomes lower, making the detection of indigo, in artworks, with emission-based techniques more challenging.
In summary, the fast and highly efficient internal conversion provides a ‘self-protective channel’, minimizing access to photodegradation. This photoprotective process is shared with other historic dyes and with DNA bases (purines and pyrimidines) [22]. For the latter, De Vries proposed that this mechanism has been preserved as a molecular fossil: "Evolution requires the existence of self-replicating molecules; the selection of the nucleobases as building blocks of those macromolecules would thus logically have taken place prior to any biological processes. Therefore, if the excited state properties have played a role in a chemical selection of today’s nucleobases, they may be a relic of prebiotic chemistry on an early earth. These photochemical properties then would be molecular fossils, so to speak, of four-billion-year-old chemistry." [22]. Molecular fossil is a powerful expression that enriches our chemical vocabulary, and discloses indigo as both a millenary dye and a molecular fossil!
2.1.2 Andean textiles and indigo photochemistry
Building on the detailed characterization of indigo photophysics we now address its degradation mechanisms in the extraordinary Andean textiles in the collection of the Museum of Fine Arts-Boston [6, 23, 24]. Pre-Columbian textiles represent the longest continuous textile record in world history and are precious and unique works of art, Figure 4 [25].
Blue colors were fully characterized from micro samples of different Andean textiles, dated from 200 B.C. – A.D. 1476 [6, 23, 24]. Here we will focus on the degradation products found in these Andean blues naturally aged over centuries. These will be compared with what observed in simulation studies in transparent gels (collagen and CMC) that reproduce the dye environment in textiles, wool (protein) and cotton (cellulose). As these are aqueous gels and indigo is insoluble in water, in these media it was replaced by it's analogous the 5,5'-indigodisulfonic acid sodium salt (indigo carmine) [6].
The reaction kinetics, in the absence and presence of oxygen, were followed by UV-visible absorption and the resulting photoproducts were characterized by HPLC-DAD-MS. Isatin was the major product observed in all the media studied [6]. Quantum yields of reaction were obtained with excitation at 335 nm and 610 nm in organic (THF, DMF) and aqueous solutions as well as in the transparent gels, BOX1. In solution, the photodegradation quantum yields ([math]\Phi _{R}[/math]) were in the order of 10[math]^{-4}[/math] and dependent on the irradiation wavelength; except for indigo carmine in water, where a [math]\Phi _{R}[/math] =9×10[math]^{-6}[/math] was obtained; in the case of indigo carmine the [math]\Phi _{R}[/math] values were found to suffer a 2-fold increase upon going from water to gels [6].
The data obtained agreed with the analysis of blues from millenary Andean textiles dated from BC 200 to 200 AD. These textiles were kept for millennia protected from light in a very dry environment [25], in a region named Paracas (South Peru). In these Paracas blues, isatin was also found as the major photodegradation product, in low to moderate relative amounts (≤10% and ≤20%), indicating that indigo is a very stable molecule in certain environments. Based on the behavior of indigo in DMF solutions [6], it was proposed that isatin is produced from a reaction of indigo with a radical, resulting from an attack to the central double bond.
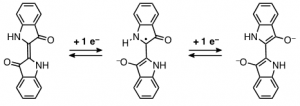
This mechanism is very plausible when considering the electrochemical studies that lead to the conversion of the keto into the leuco form, Scheme 1, involving the disruption of the central double bond connecting the two indole moieties [26]. It was proved that the mechanism was based on a two-step reduction and that the reduction potential, [math]E_{red}^{\frac{1}{2}}[/math], was pH dependent, scheme 1; therein it was suggested the possibility of a protonation step following charge transfer to be rate-limiting [27]. Further studies on the electrochemical reduction of indigo dissolved in organic solvents (DMSO, DMF) confirmed the general reduction mechanism [27]. This reduction mechanism shows the formation of a radical in one of the carbon atoms of the central double bond; this radical, in turn, will react very promptly with other radicals present in solution.
Based on all the experimental evidence collected, we propose that the central double bond is at the same time a photoprotector and a weak point. It is the backbone of the photoprotection mechanism as it enables a very fast non-radiative deactivation process from the excited to the fundamental state, "locking" the molecule. But, it is also the most reactive bond of indigo; in our photodegradation experiments, we have proved that its disruption could occur via the mechanism depicted in Scheme 1 for indigo reduction. Finally, based on the obtained [math]\Phi _{R}[/math], we propose that photodegradation quantum yields as found in water, below 10[math]^{-6}[/math], could be representative for indigo in many works of art, and that the values ranging from 10[math]^{-4}[/math] to 10[math]^{-3}[/math] could be representative of environments where the presence of oxygen based radicals or reducing species could be present.
2.2 Light as information bits: advanced analytical techniques for "attacking the actual cause" of degradation
2.2.1 The Molab approach
The Molab infrastructure initiated in the European project EU-Artech in 2004-2009 is a model of a successful approach into the study of iconic European and International artworks using portable equipment [28]. Molab proposed a holistic approach to the characterization of the work of art: enough data must be collected by in situ non-invasive methodologies to be representative of the materials and techniques of a certain work of art. Within Molab, fluorescence techniques in the visible were developed and systematically applied for the in situ study of artworks [29, 30].
Presently, the field is progressing from single point analysis to mapping small to large areas. The success of reflectance hyper spectral imaging in the VIS-NIR is an example of such a trend [31, 32].
2.2.2 Microspectrofluorimetry for the characterization of medieval pigments
As important as collecting in situ data is the ability to analyze very small areas at the micrometer range. Microspectrofluorimetry proved to be a unique technique for the study of dyes in works of art; it offers high sensitivity and selectivity, combined with good spatial resolution and fast data acquisition; it can also be used in situ without any contact with the sample or work of art to be analyzed.
The potentiality of microspectrofluorimetry for a complete characterization of the organic colorants used in the past was tested, first, in historically accurate reproductions and finally, in works of art, such as textiles, medieval illuminations or oil paintings [23, 24]. The importance of sensitivity is clear when the following facts are considered: some of the dyes used in the past to create bright colors may have faded or may have been applied as very thin coats over, or mixed with, an inorganic pigment or extender, and consequently they may be present in very low concentrations. The possibility of in situ analysis of ancient colorants is an advantage, particularly when considering that the techniques currently employed for dye analysis (HPLC-DAD-MS, microFTIR and microRaman) require micro-sampling. Microspectrofluorimetry also presents some drawbacks, namely the absence of a molecular fingerprint as disclosed in infrared spectra. This limitation may be overcome by using consistent databases build-up with historically accurate reproductions as well as by complementing it with other analytical approaches such as Fibre Optic Reflectance Spectroscopy (FORS) and Surface-enhanced Raman spectroscopy (SERS).
3 The invisible in paint: the binding medium
Oil painting and tempera painting are commonly employed for describing a painting technique in museums and exhibitions. These descriptions hide more than they tell, since oil paint formulations in the 14th or 15th century are only remotely similar to what we may find nowadays in commercial artist's materials. Nevertheless, we need to acknowledge the tremendous impact binding media have had on the history of art; proteinaceous temperas were used in the 12th century when the great art of colour was in the codex, illuminating God's word; the Flemish school masterfully used linseed oil to promote a revolution in the way nature and humans were portrayed; the 20th and 21st century have inherited the new direction that Impressionism motivated, and welcomed experimentation with new materials never seen before. Oil painting is still important but in the 21st c. water emulsions are ubiquitous.
During the 1940s and 1950s, the first artist's formulations based on vinyl and acrylic emulsions were commercialized [33] and replaced the primacy of oil paints. Photo-oxidation is considered the main factor affecting polymer weathering [34-38]. Works of art made with vinyl paints are mainly exposed to radiation filtered by glass windows ([math]\lambda \geq [/math]300 nm), and consequently no direct absorption of light by the polymer carbonyl group is expected. Radiation at [math]\lambda \geq [/math]300 nm will most likely be absorbed by chromophores in the PVAc matrix, such as hydroperoxide groups formed during synthesis or processing [37]. Even present in very low amounts, and therefore absorbing a very small fraction of light, they may act as initiation sites for polymer degradation.
For a better understanding of the photo-oxidative mechanisms of poly(vinyl acetate) homopolymer, studies under both polychromatic (Xenon-arc lamp at [math]\lambda \geq [/math]300 nm) and monochromatic (at 313 nm) radiation have been carried out. As the first indications for the molecular deterioration of the polymer are given by changes in the molecular weight distribution (e.g., scission or crosslinking), the molecular evolution is followed by infrared spectroscopy (FTIR) and size exclusion chromatography (SEC). Reaction quantum yields ([math]\Phi _{R}[/math]) constitute a universal and straightforward parameter to assess and compare polymer photostability and as such they were also calculated [36, 39, 40], BOX 2. In polymer systems, [math]\Phi _{R}[/math] is defined as the ratio between the number of molecules undergoing chain scission, crosslinking, or any other relevant photodegradation aspect per photon absorbed [38, 39], BOX 2.
Molecular evolution
The photo-oxidative mechanisms of poly(vinyl acetate) homopolymer are discussed in BOX 3. Infrared spectroscopy has shown that molecular changes are small, almost negligible. After prolonged irradiation, the intensity of the PVAc spectrum is lowered by a maximum of 10%, and its shape is maintained when compared to non-irradiated samples. Furthermore, no PVAL formation was detected [34]. On the other hand, changes in molecular weight distributions are present after 500 hours under irradiation. With no indication for crosslinking, the serial decrease in weight average molecular weight (Mw) and the slight increase in its dispersity indicate that PVAc undergoes chain scission from the beginning of the irradiation. Based on the number of scissions per chain, the [math]\Phi _{R}[/math] for PVA chomopolymer at 313 nm is 7.40[math]\times[/math]10[math]^{-8}[/math]. When compared with the values published in the literature for irradiation involving 254 nm [41], the [math]\Phi _{R}[/math] for PVAc is five orders of magnitude lower, which indicates that a different photochemical mechanism is involved [34, 41, 42].
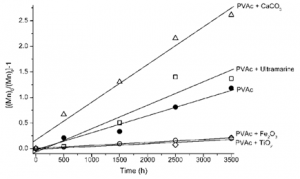
An important aspect to consider when analyzing the degradation of paints is the effect of pigments and fillers on the polymer stability [34]. Looking at the scission process in more detail, the number of scissions per chain, S, may be calculated by S = Mn[math]_{0}[/math] / Mn[math]_{t}[/math] – 1 and it is possible to separate the pigments into two groups: stabilizing and sensitizing, Figure 6. Titanium dioxide and iron oxide display a protective effect on paint films shown by a decrease in the scission rate when compared to PVAc alone. On the other hand, ultramarine blue and calcium carbonate in particular (frequently present in paints as filler) promote an increase in the polymer scission rate.
It is possible that TiO[math]_{2}[/math] and Fe[math]_{2}[/math]O[math]_{3}[/math] are sold with a protective coating, i.e. encapsulated with inert materials. In this case, it is necessary to consider the partition of light absorption between pigment and polymer. If the pigment can compete for light absorption, without promoting any secondary photochemical reactions, it will display a protective effect; the larger the fraction of light absorbed, especially for lower wavelengths, the more efficient this effect will be. Both iron oxide and titanium dioxide absorb completely (cut off) radiation below 400 nm [34]. Therefore, a protective effect is observed. In turn, the results obtained for calcium carbonate do not present an immediate explanation, but some hypothesis may be formulated. Calcium carbonate does not display a sharp cut-off, and between 300 and 350 nm both reflects and scatters light [34]. In this region, both the scattered and reflected light could be re-absorbed by the polymer (internal scattering), intensifying the light absorption [43]. A similar reasoning might explain the observation for ultramarine [44], but in this case due to the presence of kaolin as an impurity.
4 Box 1: Measuring a reaction quantum yield in a transparent gel
by Fernando Pina.
In a photochemical reaction in solution as represented in eq.(1) the quantum yield is defined by means of eq.(2)
[math]\mathbb{R}\overset{h\nu }{\rightarrow}\mathbb{P}\tag{1}[/math]
[math]\Phi =\frac{number\; of\; P\; molecules\; formed}{number\; of\;absorbed \; photons}=\frac{\Delta A_{\lambda _{m}}}{\Delta A_{\epsilon _{m}}}\nu _{irr}\left \{ I_{0}(1-10^{-A_{\lambda _{irr}}})\frac{A_{\lambda_{irr(R)}}}{A_{\lambda_{irr(R)}}+A_{\lambda_{irr(P)}}}\Delta t \right \}^{-1}\tag {2}[/math]
where [math]\Delta A_{\lambda _{m}}[/math] is the mean absorbance variation at the monitoring wavelength [math]\lambda_{m}[/math], [math]\Delta A_{\epsilon _{m}}[/math]is the difference between the molar absorption coefficients of reagent and product at the same wavelength, I0 is the intensity of the light, and [math]A_{\lambda _{irr}}=A_{\lambda _{irr(R)}}+A_{\lambda _{irr(P)}}[/math] is the total absorbance of the solution at the irradiation wavelength, where [math]A_{\lambda _{irr(R)}}=\varepsilon _{irr(R)}b\left [ R \right ][/math] and [math]A_{\lambda _{irr(P)}}=\varepsilon _{irr(P)}b\left [ P \right ][/math] are the contributions from reagent and product, respectively. The irradiation path length is [math]b[/math], while [math]\nu _{irr}[/math] the irradiated volume, is the product of the illuminated surface of the cell and the optical path. The time interval over which the absorption changes are measured is [math]\Delta t[/math]. The therm [math]I_{0}(1-10^{-A_{\lambda _{irr}}})[/math] accounts for the total light absorbed by the system (both reagent and product), while the fraction [math]\frac{A_{\lambda_{irr(R)}}}{{A_{\lambda_{irr(R)}}+A_{\lambda_{irr(P)}}}}[/math] gives the fraction that is absorbed by the reagent responsible for the photochemical events.
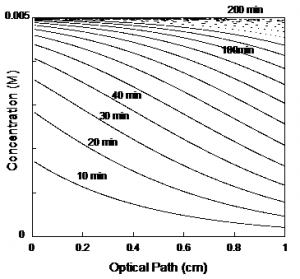
One interesting aspect of photochromic gels is the formation of a gradient of product concentration that can be observed at the naked eye in concentrated solutions absorbing in the visible. This effect is due to the penetration of light through the optical path. The gradient formation does not affect the measurement of the quantum yields, if the irradiated volume is defined by the area of the cell under light and the optical path. For more details see F. Pina, A. T. Hatton, “ Photochromic Soft Materials: Flavylium compounds Incorporated in Pluronic F-127 hydrogel Matrixes”, Langmuir, 2008, 24, 2356-2364.
In conclusion, eq.(2) can be used indistinctly in a transparent gel or in a stirred solution. However, in the case of a gel, the irradiated volume should be defined by the irradiated face and the optical pathway and the incident light should be homogeneously distributed through the surface.
5 Box 2: Measuring a reaction quantum yield ([math]\Phi_{R}[/math]) in a solid polymer matrix
The [math]\Phi_{R}[/math] for PVAc photodegradation is considered as the rate of scissions per chain, S’, per mole of photons absorbed, [math]I_{A}[/math] (eq 4). To minimize experimental error in using all the absorption spectra for [math]\lambda[/math]≥300nm, [math]\Phi_{R}[/math] is calculated for 313 nm, a wavelength in which it is still possible to measure the polymer matrix absorption with acceptable accuracy [34]. As [math]\Phi_{R}[/math] is dimensionless, the units for both S’ and [math]I_{A}[/math] should be the same (as detailed).
I[math]_{0}[/math] and I[math]_{A}[/math]
I[math]_{0}[/math] at 313 nm is obtained using potassium hexacyanocobaltate(III) as actinometer and eq. 1; I[math]_{0}[/math] is expressed as mol.cm[math]^{-2}[/math].min[math]^{-1}[/math], i.e., the mole of photons per unit area per unit time.
[math]I_{0}=\frac{V_{sol}\times \left ( \frac{\Delta A}{\Delta \varepsilon } \right )}{1000\times \Phi _{R}\times \Delta t}=1.16 10^{-7}mol.L^{-1}.cm.min^{-1}=1.16 10^{3}.mol.cm^{-2}.min^{-1}\tag {1}[/math]
where, V[math]_{sol}[/math] is the volume of irradiated solution in ml (3ml); [math]\Delta A[/math] is the change in absorbance at the monitoring wavelength (380 nm) over the irradiation time period, corrected by the light absorption of the reagent at 313 nm; [math]\Delta \varepsilon [/math] is the difference between the molar absorption coefficients of reagent ([math]\epsilon _{R}[/math]=10 M[math]^{-1}[/math]cm[math]^{-1}[/math]) and product ([math]\epsilon _{R}[/math]=280 M[math]^{-1}[/math]cm[math]^{-1}[/math])at the monitoring wavelength, in L.mol[math]^{-1}[/math].cm[math]^{-1}[/math]; 1000 corresponds to ml; [math]\Delta t[/math] to irradiation period in min; and [math]\Phi _{R}[/math] is the quantum yield of the reaction ([math]\Phi _{R}[/math]=0.31) [34].
The number of photons absorbed is: [math]I_{A}=I_{0}\times \left ( 1-10^{-A} \right )=1.16\times 10^{-4}\times (1-10^{-0.0155})=4.1\times 10^{-6}mol.cm^{-2}.min^{-1}\tag{2}[/math]
Rate of scissions per chain
The number of scissions per chain per unit time (S’) was determined as 2.0x10[math]^{-6}[/math]. To be used to calculate [math]\Phi_{R}[/math] this number must refer to the moles irradiated per area, i.e., it must be multiplied by the weight of polymer irradiated (w, g) and divided by Mn[math]_{0}[/math] and irradiated area (a, cm[math]^{2}[/math]), as indicated below:
[math]\frac{S^{'}\times w}{Mn_{0}\times a}=\frac{2.0\times 10^{-6}\times 0.01981}{39982\times 3.3}=3.0\times 10^{-13}mol.cm^{-2}.min^{-1}\tag{3}[/math]
Quantum yield
The [math]\Phi_{R}[/math] may now be obtained by dividing the moles of polymer undergoing chain scission (eq 3) by the moles of photons absorbed, both by unit area and unit time:
[math]\Phi _{R}(PVAc)=\frac{3.0\times 10^{-13}}{4.1\times 10^{-6}}=7.40\times 10^{-8}\tag{4}[/math]
6 Box 3: Norrish reaction mechanisms in PVAc photodegradation
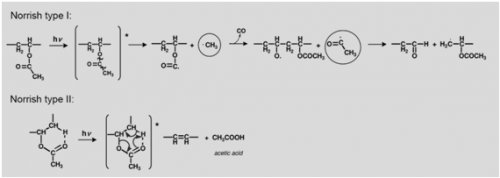
The formation of acetic acid, carbon monoxide and other volatile molecules observed upon irradiation at 254 nm [61] may be explained based on an heterolytic process or / and via an homolytic mechanism based on Norrish type I reactions, Figure 5. Heterolytic bond scission may occur as a Norrish type II mechanism or an ester hydrolysis that will lead to the formation of acetic acid and poly(vinyl alcohol).
It has been shown that upon PVAc irradiation at 254 nm acetic acid is formed as the main product with a quantum yield of reaction of 10[math]^{-2}[/math] [38]. This would be extremely dangerous in a museum environment, because acetic acid reacts with innumerous materials in works of art, such as pigments and metals where lead is present. As photochemists we are aware that a reaction activated by light at 254 nm may or may not occur when the same sample is irradiated at 313 nm. A work of art may be found indoor or outdoor and in neither case it will be possible for a material to absorb at 254 nm, as this part of the solar spectrum does not reach the Earth’s surface [54]. So in our set-up we will mimic the photodegradation that may occur upon natural ageing by irradiating PVAc paints with light filtered at 300 nm or monochromatic 313 nm radiation. We now will take a closer look on the processes leading to the formation of acetic acid to demonstrate how they may be monitored by infrared spectroscopy.
Acetic acid may be formed by heterolytic (non-photochemical) acidic hydrolysis, resulting in the formation of poly(vinyl alcohol), which, according to our previous studies, is detected in the infrared spectrum for a concentration of PVAL greater than 10% [57]. The appearance of an O-H stretching from the alcohol group present in PVAL together with the ratios [math]\nu[/math]CH[math]_{3}[/math]/C=O, [math]\nu[/math]CH[math]_{2}[/math]/C=O, [math]\delta[/math]CH[math]_{3}[/math]C=O and [math]\nu[/math]C-O/C=O provide relevant information about the extent and type of the ester fragmentation.
In a Norrish Type II mechanism, Figure 5, acetic acid release is accompanied by the formation of a double bond in the main chain. Due to its low molar absorptivity the formation of these double bonds might not be detected in the infrared spectrum, but the extensive production of acetic acid would be reflected ina decrease of polymer weight; it would also be detectable in the infrared spectra by a loss of intensity as well as a broadening of the ester absorption band.
In a Norris type I, besides the changes in the carbonyl absorption just described, the side chain reactions are readily observed in the infrared spectrum; the loss of a CH[math]_{3}[/math] group would be monitored both in the C=O/C-H ratios and in alteration of the CH[math]_{3}[/math] stretching and CH[math]_{3}[/math] bending, at approximately 2970 cm[math]^{-1}[/math] and 1373 cm[math]^{-1}[/math], respectively.
7 References
[1] Balzani, Vincenzo, and Franco Scandola. "Supramolecular Photochemistry 1991." Horwood, Chichester (1994).
[2] Kroustallis, Stefanos. "Binding media in medieval manuscript illumination: a source research." Revista de Historia da Arte 1, no. Serie W (2011): 113-125.
[3] Melo, Maria J., Rita Castro, and Adelaide Miranda. "Colour in medieval portuguese manuscripts: Between beauty and meaning." Science and art: the painted surface (eds A Sgamellotti, BG Brunetti, C Miliani) (2014): 170-192.
[4] Balfour-Paul, Jenny. Indigo. Vol. 264. London: British Museum Press, 1998.
[5] Seixas de Melo, J. Sérgio, A. P. Moura, and M. J. Melo. "Photophysical and spectroscopic studies of indigo derivatives in their keto and leuco forms." The Journal of Physical Chemistry A 108, no. 34 (2004): 6975-6981.
[6] Sousa, Micaela M., C. Miguel, I. Rodrigues, A. Jorge Parola, F, Pina, J. S. Seixas de Melo, and M. J. Melo. "A photochemical study on the blue dye indigo: from solution to ancient Andean textiles." Photochemical &Photobiological Sciences 7, no. 11 (2008): 1353-1359.
[7] Melo, Maria J. "History of natural dyes in the ancient mediterranean world." Handbook of natural colorants (2009): 3-20.
[8] Cooksey, Christopher J. "Tyrian purple: 6, 6’-dibromoindigo and related compounds." Molecules 6, no. 9 (2001): 736-769.
[9] Wyman, George M. "Reminiscences of an accidental Photochemist." EPA News Lett 50, no. 9 (1994).
[10] Bauer, Helmut, K.Kowski, H. Kuhn, W.Lüttke, and P.Rademacher. "Photoelectron spectra and electronic structures of some indigo dyes." Journal of molecular structure 445, no. 1-3 (1998): 277-286.
[11] Wille, E., and W. Luttke. " Theoretical and spectroscopic studies on Indigo Dyes. 9. 4,4,4’,4’-Tetramethyl-Delta2,2’-Bipyrrolidine-3,3’-Dione, a compound having basic chromophore system of Indigo." Angewandte Chemie-International Edition 10, no. 11 (1971): 803.
[12] Elsaesser, T., W. Kaiser, and W. Lüttke. "Picosecond spectroscopy of intramolecular hydrogen bonds in 4, 4', 7, 7'-tetramethylindigo." The Journal of Physical Chemistry 90, no. 13 (1986): 2901-2905.
[13] Klessinger, Martin. "The origin of the colour of indigo dyes." Dyes and Pigments 3, no. 2-3 (1982): 235-241.
[14] Klessinger, Martin. "Captodative substituent effects and the chromophoric system of Indigo." Angewandte Chemie International Edition 19, no. 11 (1980): 908-909.
[15] Jacquemin, Denis, J.Preat, V.Wathelet, and E. A. Perpète. "Substitution and chemical environment effects on the absorption spectrum of indigo." The Journal of chemical physics 124, no. 7 (2006): 074104.
[16] Miliani, Costanza, A. Romani, and G. Favaro. "A spectrophotometric and fluorimetric study of some anthraquinoid and indigoid colorants used in artistic paintings." SpectrochimicaActa Part A: Molecular and Biomolecular Spectroscopy 54, no. 4 (1998): 581-588.
[17] Amat Anna, F. Rosi, C. Miliani, A. Sgamellotti, and S. Fantacci. “Theoretical and experimental investigation on the spectroscopic properties of indigo dye, Journal of Molecular Structure n° 993 (2011) 43–51
[18] Kobayashi, T., andP. M. Rentzepis. "On the picosecond kinetics and photostability of indigo and 6, 6′‐dimethoxyindigo." The Journal of Chemical Physics 70, no. 2 (1979): 886-892.
[19] Seixas de Melo, J. Sérgio, H. D. Burrows, C.Serpa, and L. G. Arnaut. "The triplet state of indigo." AngewandteChemie International Edition46, no. 12 (2007): 2094-2096.
[20] Seixas de Melo, J., R. Rondão, H. D. Burrows, M. J. Melo, S.Navaratnam, R.Edge, and G.Voss. "Spectral and photophysical studies of substituted indigo derivatives in their keto forms." ChemPhysChem7, no. 11 (2006): 2303-2311.
[21] a) Nagasawa, Yutaka, R.Taguri, H. Matsuda, Ma. Murakami, M.Ohama, T. Okada, and H.Miyasaka. "The effect of hydrogen-bonding on the ultrafast electronic deactivation dynamics of indigo carmine." Physical Chemistry Chemical Physics 6, no. 23 (2004): 5370-5378.; b) I., Izumi, A.Yabushita, and T. Kobayashi. "Transition state in a prevented proton transfer observed in real time." Bulletin of the Chemical Society of Japan 84, no. 2 (2011): 164-171.
[22] Kleinermanns, Karl, D.Nachtigallová, and M. S. de Vries. "Excited state dynamics of DNA bases." International Reviews in Physical Chemistry32, no. 2 (2013): 308-342.
[23] Claro, Ana, M. J. Melo, J. S.Seixas de Melo, K. J. van den Berg, A.Burnstock, M. Montague, and R. Newman. "Identification of red colorants in van Gogh paintings and ancient Andean textiles by microspectrofluorimetry." Journal of Cultural Heritage 11, no. 1 (2010): 27-34
[24] Melo, Maria J., and A. Claro. "Bright light: microspectrofluorimetry for the characterization of lake pigments and dyes in works of art." Accounts of chemical research 43, no. 6 (2010): 857-866.
[25] Paul, Anne. Paracas art & architecture: object and context in south coastal Peru. University of Iowa Press, 1991.
[26] Srividya, N., G. Paramasivan, K. Seetharaman, and P. Ramamurthy. "Two-step reduction of indigo carmine by dithionite: a stopped-flow study." Journal of the Chemical Society, Faraday Transactions 90, no. 17 (1994): 2525-2530.
[27] Bond, Alan M., F.Marken, E. Hill, R. G. Compton, and H.Hügel. "The electrochemical reduction of indigo dissolved in organic solvents and as a solid mechanically attached to a basal plane pyrolytic graphite electrode immersed in aqueous electrolyte solution." Journal of the Chemical Society, Perkin Transactions 2 9 (1997): 1735-1742.
[28] Miliani, Costanza, F.Rosi, B. G.Brunetti, and A.Sgamellotti. "In situ noninvasive study of artworks: the MOLAB multitechnique approach." Accounts of chemical research 43, no. 6 (2010): 728-738.
[29] Romani, Aldo, C.Clementi, C.Miliani, and G.Favaro. "Fluorescence spectroscopy: A powerful technique for the noninvasive characterization of artwork." Accounts of chemical research 43, no. 6 (2010): 837-846.
[30]Brunetti, Brunetto G., Miliani, C., Doherty B., Monico, L., Romani, A., and Sgamellotti, A. "Non-invasive Investigations of Paintings by Portable Instrumentation: The MOLAB Experience." Topics in Current Chemistry(J) 374, no. 1 (2016): article number 10.
[31] Cucci, Costanza, J. K. Delaney, and M.Picollo. "Reflectance hyperspectral imaging for investigation of works of art: old master paintings and illuminated manuscripts." Accounts of chemical research 49, no. 10 (2016): 2070-2079.
[32] Vitorino, T., A. Casini, C. Cucci, M. J. Melo, M. Picollo, and L. Stefani. "Non-invasive identification of traditional red lake pigments in fourteenth to sixteenth centuries paintings through the use of hyperspectral imaging technique." Applied Physics A 121, no. 3 (2015): 891-901.
[33] Crook, Jo, and Tom Learner. The impact of modern paints. Tate Gallery Pub., 2000.
[34] Ferreira, Joana L., M. J. Melo, and A. M. Ramos. "Poly (vinyl acetate) paints in works of art: a photochemical approach. Part 1." Polymer Degradation and Stability 95, no. 4 (2010): 453-461.
[35] Lemaire, Jacques, Jean-Luc Gardette, J. Lacoste, P.Delprat, and D.Vaillant. "Mechanismsof photooxidation of polyolefins: prediction of lifetime in weathering conditions." In Polymer Durability: degradation, stabilization and lifetime predictions,edited by R. L. Clough, N. C. Billingham, K. T. Gillen, 577-98. American Chemical Society, 1996.
[36] Pospíšil, J., J. Pilař, N. C. Billingham, A. Marek, Z. Horak, and S. Nešpůrek. "Factors affecting accelerated testing of polymer photostability." Polymer Degradation and Stability 91, no. 3 (2006): 417-422.
[37] Allen, Norman S., and M. Edge. Fundamentals of polymer degradation and stabilization. Springer Science & Business Media, 1992.
[38] Rabek, Jan F. Polymer photodegradation: mechanisms and experimental methods. Chapman & Hall, 1995.
[39] Fox, Robert B. "Photodegradation of high polymers." Progress in polymer science 1 (1967): 45-89.
[40] Montalti, Marco, Alberto C., L. Prodi, and M. Teresa Gandolfi. Handbook of photochemistry. CRC press, 2006.
[41] Benavides, R., R. González‐Hernandez, M. C. González‐Cantú, B. Reyes‐Vielma, and N. C. Billingham. "Accelerated degradation of highly loaded polypropylene." Journal of Vinyl and Additive Technology 9, no. 1 (2003): 41-49.
[42] Rivaton, Agnès, Jean‐Luc Gardette, B.Mailhot, and S.Morlat‐Therlas. "Basic aspects of polymer degradation." In Macromolecular Symposia, vol. 225, no. 1, pp. 129-146. WILEY‐VCH Verlag, 2005.
[43] Buchanan, K. J., and W. J. McGill. "Photodegradation of poly (vinyl esters)—II. Volatile product formation and changes in the absorption spectra and molecular mass distributions." European Polymer Journal 16, no. 4 (1980): 313-318.
[44] Melo, Maria J., Joana L. Ferreira, António J. Parola and João Seixas de Melo, "Photochemistry for Cultural Heritage." In Applied Photochemistry: When Light Meets Molecules, edited by Serena Silvi, Giacomo Bergamini, 499-530. Netherlands: Springer, 2016.